Dr. Chi-Kong Yeung BSc, PhD, CBiol, FSB , FIBMSAdjunct Associate Professor
Room 704 and Room 313 (Microelectrode Array Laboratory) Lo Kwee-Seong Integrated Biomedical Sciences Building Faculty of Medicine Chinese Univesity of Hong Kong Shatin, N.T. Hong Kong Tel: +852 9533 2699 e-mail: [email protected] See Also: http://jar-labs.vomifix.com/prof-ck-yeung.html http://www2.sbs.cuhk.edu.hk/en-gb/people/academic-staff/prof-rudd-john-anthony http://www2.sbs.cuhk.edu.hk/en-gb/people/honorary-staff |
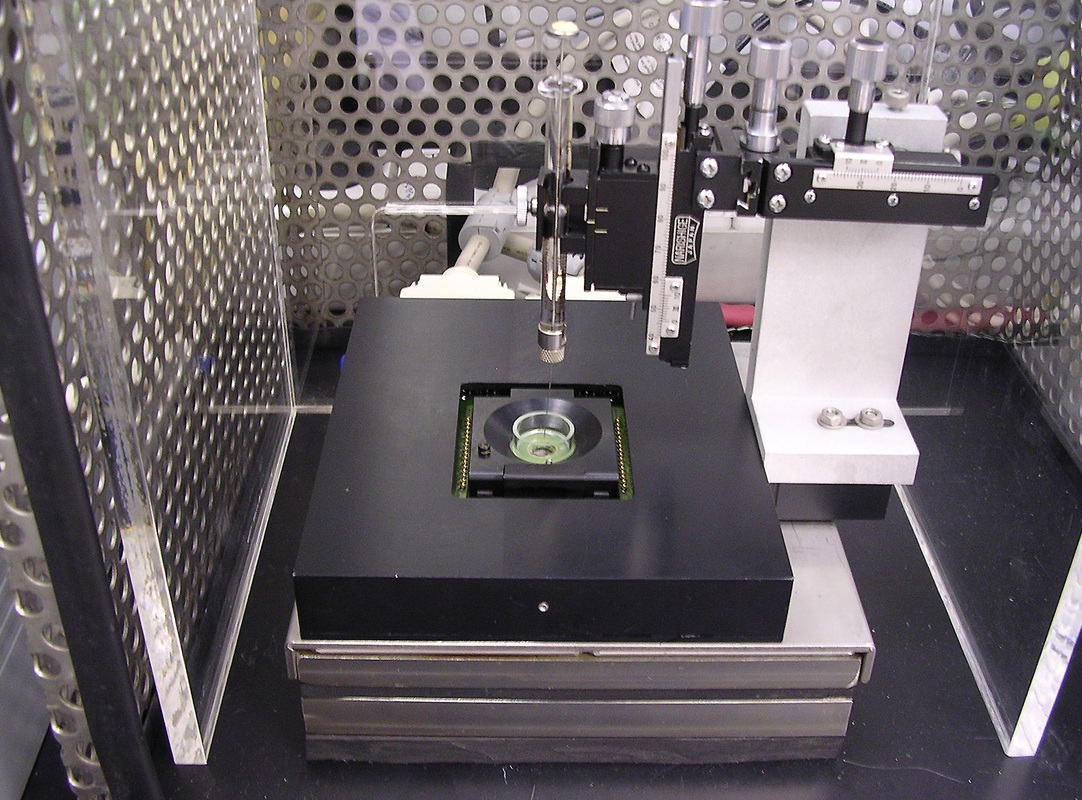
Microelectrode Array Laboratory
Education
1996-1997 Certificate in Teaching and Learning in Higher Education
1993-1996 PhD in Pharmacology/Physiology
1990-1993 BSc (Hons) in Biomedical Sciences (Specialising in Pharmacology & Toxicology)
1993-1996 PhD in Pharmacology/Physiology
1990-1993 BSc (Hons) in Biomedical Sciences (Specialising in Pharmacology & Toxicology)
Professional Memberships
Since 2013 FIBMS - Fellow of the Institute of Biomedical Science (UK)
Since 2010 FSB - Fellow of the Society of Biology (UK)
Since 2001 CBiol - Chartered Biologist (UK)
2004 - 2013 MIBMS - Member of the Institute of Biomedical Science (UK)
2001 - 2010 MBiol - Member of the Society of Biology (UK) (formerly the Institute of Biology)
Since 2010 FSB - Fellow of the Society of Biology (UK)
Since 2001 CBiol - Chartered Biologist (UK)
2004 - 2013 MIBMS - Member of the Institute of Biomedical Science (UK)
2001 - 2010 MBiol - Member of the Society of Biology (UK) (formerly the Institute of Biology)
Current Positions
Adjunct Associate Professor, School of Biomedical Sciences, Faculty of Medicine, Chinese University of Hong Kong.
Guest Professor, SOC National Laboratories (China)
Guest Lecturer, Department of Ophthalmology and Visual Sciences, Faculty of Medicine, Chinese University of Hong Kong.
Guest Professor, SOC National Laboratories (China)
Guest Lecturer, Department of Ophthalmology and Visual Sciences, Faculty of Medicine, Chinese University of Hong Kong.
Previous Positions
Assistant Professor, Bioengineering Graduate Program, Hong Kong University of Science and Technology.
Research Associate, Department of Ophthalmology and Visual Sciences, Faculty of Medicine, Chinese University of Hong Kong.
Pharmacologist/Cell Biologist (Consultant), Defence Evaluation and Research Agency (DERA, Winfrith, now known as QinetiQ). Dorchester, Dorset, UK.
Postdoctoral Researcher (SONY-funded), Max-Planck-Institute for Polymer Research (MPI-P), Ackermannweg 10, Mainz, D-55128. Germany.
Postdoctoral Researcher (MRC-funded, UK),The School of Pharmacy, Postgraduate Studies in Pharmacology, University of Bradford, UK.
Visiting Scientist, The Institute of Physical and Chemical Research (RIKEN). Frontier Research Program. Laboratory for Exotic Nanomaterials, Wako, Saitama, Japan.
Research Associate, Department of Ophthalmology and Visual Sciences, Faculty of Medicine, Chinese University of Hong Kong.
Pharmacologist/Cell Biologist (Consultant), Defence Evaluation and Research Agency (DERA, Winfrith, now known as QinetiQ). Dorchester, Dorset, UK.
Postdoctoral Researcher (SONY-funded), Max-Planck-Institute for Polymer Research (MPI-P), Ackermannweg 10, Mainz, D-55128. Germany.
Postdoctoral Researcher (MRC-funded, UK),The School of Pharmacy, Postgraduate Studies in Pharmacology, University of Bradford, UK.
Visiting Scientist, The Institute of Physical and Chemical Research (RIKEN). Frontier Research Program. Laboratory for Exotic Nanomaterials, Wako, Saitama, Japan.
Research Interests
1. Physiology and Pharmacology of the Gastrointestinal Tract and Smooth Muscles: Potassium channels in the gastrointestinal tract and vascular smooth muscle (in vitro and in vivo models).
2. Cell-Based Biosensors, Pharmacology and Physiology, Drug Profiling, Disease Models (cardiac and neuronal hypoxia) in vitro: Electrophysiology, cell-integrated microelectrode arrays (MEAs) and field effect transistors in pharmacological and biomedical research, and disease model development.
3. Neuroscience and Cell Guidance: Micro-contact printing, neuronal cell patterning/organised formation of live neural network, synaptic formation, and neural toxicity.
4. Ophthalmology and Vision Research: Corticosteroids and other substances (e.g. Traditional Chinese Medicines) on retinal pigment epithelial cells, in vivo model of myopia, cell and molecular biology of the eye, cell transplantation, and toxicology of the eye.
Specific Projects
(1) Cardiac Pathophysiology
Two ischaemia models have already been established on the MEA for the study of the effects of KATP channels, especially mitoKATP and sarcKATP channels, on compromised cardiac functions and the significance of preconditioning. This research direction helps us to understand the processes involved in cardiac myocyte functions, and how we can alleviate the physiological changes that take place in ischaemia and hypoxia.
(2) Live Neural Network
The assembly of cultured mammalian neurons can help us unravel how neurons are organised, and whether the application of certain pharmacological agents could enhance synaptic formation. With this in mind, special chips that can ‘manipulate’ neurite outgrowth and synaptic formation (i.e. memory and strength of connectivity) have been designed and manufactured. Using the experience gained in cardiac ischaemia, I will also be examining ways to alleviate neuronal hypoxic damage.
(3) Gastrointestinal Motility
My PhD studies of how potassium channels affect GI motility involved the use of both in vitro and in vivo approach. Recently, I have again begun working on this important aspect of intestinal function using radiotelemetry, which provides very important physiological data such as wavefront propagation, as well as heart rate and body temperature that are often associated with emetic reflex.
(4) Immunopharmacology
I am the first to incorporate the use of MEA in the study of mast cell degranulation and histamine release (induced by compound 48/80, anti-IgE) and its relationship with potassium channel modulators (e.g. pinacidil, diazoxide, glibenclamide) and calcium channel blockers (e.g. verapamil and nifidepine). This research theme enables us to look into the control of immunological responses and how we can better control allergies such as asthma. We are currently in the process of applying an industrial grant for this line of research.
2. Cell-Based Biosensors, Pharmacology and Physiology, Drug Profiling, Disease Models (cardiac and neuronal hypoxia) in vitro: Electrophysiology, cell-integrated microelectrode arrays (MEAs) and field effect transistors in pharmacological and biomedical research, and disease model development.
3. Neuroscience and Cell Guidance: Micro-contact printing, neuronal cell patterning/organised formation of live neural network, synaptic formation, and neural toxicity.
4. Ophthalmology and Vision Research: Corticosteroids and other substances (e.g. Traditional Chinese Medicines) on retinal pigment epithelial cells, in vivo model of myopia, cell and molecular biology of the eye, cell transplantation, and toxicology of the eye.
Specific Projects
(1) Cardiac Pathophysiology
Two ischaemia models have already been established on the MEA for the study of the effects of KATP channels, especially mitoKATP and sarcKATP channels, on compromised cardiac functions and the significance of preconditioning. This research direction helps us to understand the processes involved in cardiac myocyte functions, and how we can alleviate the physiological changes that take place in ischaemia and hypoxia.
(2) Live Neural Network
The assembly of cultured mammalian neurons can help us unravel how neurons are organised, and whether the application of certain pharmacological agents could enhance synaptic formation. With this in mind, special chips that can ‘manipulate’ neurite outgrowth and synaptic formation (i.e. memory and strength of connectivity) have been designed and manufactured. Using the experience gained in cardiac ischaemia, I will also be examining ways to alleviate neuronal hypoxic damage.
(3) Gastrointestinal Motility
My PhD studies of how potassium channels affect GI motility involved the use of both in vitro and in vivo approach. Recently, I have again begun working on this important aspect of intestinal function using radiotelemetry, which provides very important physiological data such as wavefront propagation, as well as heart rate and body temperature that are often associated with emetic reflex.
(4) Immunopharmacology
I am the first to incorporate the use of MEA in the study of mast cell degranulation and histamine release (induced by compound 48/80, anti-IgE) and its relationship with potassium channel modulators (e.g. pinacidil, diazoxide, glibenclamide) and calcium channel blockers (e.g. verapamil and nifidepine). This research theme enables us to look into the control of immunological responses and how we can better control allergies such as asthma. We are currently in the process of applying an industrial grant for this line of research.
|
Click for Specific Projects
|
Grants
1. Germany/Hong Kong Joint Research Scheme: Co-I, To Study the Electrophysiological Response of Human Mast Cell Activation and Their Implication in the Treatment of Allergies Using Microelectrode Array (MEA)’. HK$57,400 (2012).
2. Germany/Hong Kong Joint Research Scheme: Co-I, ‘To study the relationship between ischaemic preconditioning and adenosine triphosphate-sensitive potassium (KATP) channels on cultured cardiac myocytes using the microelectrode array (MEA).’ HK$57,400 (2009).
3. Direct Grant: Co-I, ‘Interstitial Cells of Cajal networking in the senescence-accelerated mouse’. HK$ 32,895 (2007).
4. Direct Grant: Co-I, ‘Determination of the therapeutic index of fluvoxamine in Suncus Murinus (House Musk Shrew)’. HK$ 50,000 (2007).
5. RGC Earmarked Research Grant: Co-I, ‘Micro-Electro-Array Technology for Cell Level Biological Signal’. M. Chan (PI), P. KO, C.K. Yeung (2006). The ranking of the proposal: Excellent. Total amount of $1,021,841. (2006).
6. Hong Kong Society of Nephrology: Co-I, ‘Pharmacokinetic Profile of Intraperitoneal cefepime in patients with CAPD peritonitis’. HK$ 30,000 (2005).
7. The Royal Society Research Visit Grant (1999) – Visiting Scientist to RIKEN, the Laboratory for Exotic Nano-Materials, Frontier Research Program, Wako, Saitama, Japan £ 1,200. (Additional support from Prof. Wolfgang Knoll of Max-Planck-Institute for Polymer Research, Germany).
2. Germany/Hong Kong Joint Research Scheme: Co-I, ‘To study the relationship between ischaemic preconditioning and adenosine triphosphate-sensitive potassium (KATP) channels on cultured cardiac myocytes using the microelectrode array (MEA).’ HK$57,400 (2009).
3. Direct Grant: Co-I, ‘Interstitial Cells of Cajal networking in the senescence-accelerated mouse’. HK$ 32,895 (2007).
4. Direct Grant: Co-I, ‘Determination of the therapeutic index of fluvoxamine in Suncus Murinus (House Musk Shrew)’. HK$ 50,000 (2007).
5. RGC Earmarked Research Grant: Co-I, ‘Micro-Electro-Array Technology for Cell Level Biological Signal’. M. Chan (PI), P. KO, C.K. Yeung (2006). The ranking of the proposal: Excellent. Total amount of $1,021,841. (2006).
6. Hong Kong Society of Nephrology: Co-I, ‘Pharmacokinetic Profile of Intraperitoneal cefepime in patients with CAPD peritonitis’. HK$ 30,000 (2005).
7. The Royal Society Research Visit Grant (1999) – Visiting Scientist to RIKEN, the Laboratory for Exotic Nano-Materials, Frontier Research Program, Wako, Saitama, Japan £ 1,200. (Additional support from Prof. Wolfgang Knoll of Max-Planck-Institute for Polymer Research, Germany).
Awards
1. Action for Vision, Young Research of the Year Award (2003) for the research on Ophthalmology and Visual Sciences (Second Runner-Up)
2. Best Poster Presentation Award, International Symposium on Ophthalmology (ISO) (2003).
2. Best Poster Presentation Award, International Symposium on Ophthalmology (ISO) (2003).
Patents
1. “Device with Recessed Tracks for Forming a Cellular Network”. International Patent Application No. PCT/GB2002/004862-P3245. QinetiQ Ltd (formerly the Defence Evaluation and Research Agency, UK).
2. “A Method of Forming a Cell Pattern on a Surface” European Patent Application No. EP00 122 915.2. SONY International (Europe) GmbH. Garching Innovation GmbH (representing Max-Planck-Society).
2. “A Method of Forming a Cell Pattern on a Surface” European Patent Application No. EP00 122 915.2. SONY International (Europe) GmbH. Garching Innovation GmbH (representing Max-Planck-Society).
Published Books
[1] ‘Writing Made Easy – A User Friendly Guide to English Writing’, 3rd Ed – ISBN 978-988-15221-4-6
[2] ‘Common English Errors Explained’, 2nd Ed – ISBN 978-988-15221-1-5
[3] 'Stop Making Errors - 101 Most Important Errors that You Must Know' – ISBN 978-988-15222-7-6
[2] ‘Common English Errors Explained’, 2nd Ed – ISBN 978-988-15221-1-5
[3] 'Stop Making Errors - 101 Most Important Errors that You Must Know' – ISBN 978-988-15222-7-6
Editorial Board Member
Journal of Biological Research, Hong Kong
Edorium Journal of Biomedical Science
Edorium Journal of Biomedical Science
Publications (From 2001 to Present)
Full Papers (*denotes corresponding author)
|
2018
1. Wang H, Lu Z, Liu YH, Sun Y, Tu L, Ngan MP, Yeung CK, Rudd JA. Establishment of a Radiotelemetric Recording Technique in Mice to Investigate Gastric Slow Waves: Modulatory Role of Putative Neurotransmitter Systems. Exp. Physiol. (In Press) 2017 2. Lu Z, Yeung CK, Lin G, Yew DTW, Andrews PLR, Rudd JA. Centrally located GLP-1 receptors modulate gastric slow waves and cardiovascular function in ferrets consistent with the induction of nausea. Neuropeptides, 65:28-36. 3. Lu Z, Yeung CK, Lin G, Yew DT, Andrews PL, Rudd JA.Insights into the central pathways involved in the emetic and behavioural responses to exendin-4 in the ferret. Auton Neurosci. S1566-0702(16)30181-3. 2014 4. Lu Z, Percie Du Sert N, Chan SW, Yeung CK, Lin G, Yew DT, Andrews PL, Rudd JA. Differential hypoglycaemic, anorectic, autonomic and emetic effects of the glucagon-like peptide receptor agonist, exendin-4, in the conscious telemetered ferret. J Transl Med. 2014 Dec 10;12(1):327 5. Chan S.W., Lu Z, Lin G., Yew D.T., Yeung C.K., Rudd J.A. (2014). The differential antiemetic properties of GLP-1 receptor antagonist, exendin (9-39) in Suncus murinus (house musk shrew). Neuropharmacology, 83:71-8. |
| ||||||||||||||||||||||||
20136. Chan S.W., Lin G, Yew D.T.W., Yeung C.K., Rudd J.A. (2013). Separation of emetic and anorexic responses of exendin-4, a GLP-1 receptor agonist in Suncus murinus (house musk shrew). Neuropharmacology 70C:141-147.
|
| ||||||
20127. Guo J., Yuan J., Huang J., Law J.K.Y., Yeung C.K., Chan M. (2012). 32.9nV/rt Hz – 60.6dB THD Dual-Band Micro-Electrode Array Signal Acquisition IC. IEEE Journal of Solid-State Circuits. 47, 1209-1220.
8. Law J.K.Y., *Yeung C. K., Frisch J., Knapp S., Ingebrandt S., Rudd J. A., Chan M. (2012). Cardioprotective effects of potassium channel openers on rat atria and isolated hearts under acute hypoxia. J Phys Pharm Adv. 2:41-48. 9. Law J.K.Y, *Yeung C.K., Lin Li., Rudd J.A., Ingebrandt S., Chan M. (2012). The use of SU-8 topographical guided microelectrode array in measuring extracellular field potential propagation. Ann Biomed Eng. 40:619-627. |
| ||||||||||||||||||
201110. Guo J., Yuan J., Huang J., Law J.K.Y., Yeung C.K., Chan M. (2011). Highly accurate dual-band cellular field potential acquisition for brain-machine interface. IEEE Journal on Emerging and Selected Topics in Circuits and Systems. 1, 461-468
11. Law J.K.Y., *Yeung C.K., Wan S.P., Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2011). The significance of chloride in the inhibitory action of disodium cromoglycate on immunologically-stimulated rat peritoneal mast cells. Biochimica et Biophysica Acta. 1810, 867-874.7 |
| ||||||||||||
201012. Chu K.M., Ngan M.P., Wai M.K., Yeung C.K., Andrews P.L.R., Percie du Sert N., Rudd J.A. (2010). Olvanil, a Non-Pungent Vanilloid Enhances the Gastrointestinal Toxicity of Cisplatin in the Ferret. Toxicol. Lett. 192, 402-407.
13. Law J.K.Y., *Yeung C.K., Yiu K.L., Rudd J.A., Ingebrandt S., Chan M. (2010). A study of the relationship between pharmacological preconditioning and adenosine triphosphate-sensitive potassium (KATP) channels on cultured cardiomyocytes using the microelectrode array. J Cardiovasc Pharmacol 56, 60-68. 14. Chu K.M., Ngan M.P., Wai M.K., Yeung C.K., Andrews P.L.R., Percie du Sert N., Rudd J.A. (2010). Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret. Neuropharmacology. 58, 383-391. |
| ||||||||||||||||||
|
| ||||||||||||||||||
200818. *Yeung C.K., Law J.K.Y., Sam S.W., Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2008). The use of microelectrode array (MEA) to study rat peritoneal mast cell activation. J Pharmacol Sci. 107, 201-212.
|
| ||||||
2007
19. Yeung C.K., Sommerhage F., Offenhäusser A., Chan M., Ingebrandt S. (2007). Drug profiling using planar microelectrode arrays. Anal Bioanal Chem. 387, 2673-2680.
|
| ||||||
200520. Kwok A.K.H., Lai T.Y.Y., Yeung C.K., Yeung Y.S., Li W.W.Y., Chiang S.W.Y. (2005). The effects of indocyanine green and endoillumination on rabbit retina: an electroretinographic and histologic study. Br.J.Ophthalmol. 89(7), 897-900.
21. Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2005). Neuron-Transistor Coupling: Interpretation of individual extracellular recorded signals. European Biophysics J, 34, 144-154. 22. Leung C., *Yeung C.K., Chiang S.W.Y., Chan K.P., Pang C.P., Lam D.S.C. (2005). GABAA and GABAC (GABAA0r) receptors affect ocular growth and form-deprivation myopia. J. Toxicol-Cutan Ocul. 24, 187-196. 23. *Yeung C.K., Chiang S.W.Y., Chan K.P., Pang C.P., Lam D.S.C. (2005). Potassium channel openers reduce the caspase-3 expression of triamcinolone-treated retinal pigment epithelial (APRE19) cells. J Toxicol-Cutan Ocul. 24, 217-226. |
| ||||||||||||||||||||||||
200424. *Yeung C.K., Chiang S.W.Y., Pang C.P., Lam D.S.C. (2004). The damaging effect of systemic injection of monosodium glutamate (MSG) on the development of normal and form deprived eyes of the chick. J. Toxicol-Cutan Ocul. 23(1), 41-52.
25. Yeung C.K., Chiang S.W.Y., Pang C.P., Lam D.S.C. (2004). Retinoic acid reduces ocular elongation in chicks with form-deprivation myopia. J Toxicol-Cutan Ocul. 23(1), 53-64. 26. Yeung C.K., Chan K.P., Chan K.M., Pang C.P., Lam D.S.C. (2004). Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J. Opthalmol. 48(3), 236-242. 27. *Yeung C.K., Chan K.P., Chiang S.W.Y., Pang C.P., Ko W.H., Lam D.S.C. (2004). Alterations of calcium homeostasis affect the survival of human retinal epithelial cells. J Toxicol-Cutan Ocul. 23(2), 135-147. 28. *Yeung C.K., Chiang S.W.Y., Chan K.P., Pang C.P., Lam D.S.C. (2004). The transfer of ocular cells using collagen. Cell Transplant. 13, 585-594. 29. Kwok A.K.H., Yeung C.K., Lai T.Y.Y., Chan K.P., Pang C.P. (2004). Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Brit J. Ophthalmol. 88(12), 1590-1594. 30. *Yeung C.K., Chiang S.W.Y., Chan K.P., Pang C.P, Lam D.S.C. (2004). Lowering intracellular calcium concentration can reduce the cytotoxicity of triamcinolone on human retinal pigment epithelial (ARPE19) cells. J Toxicol-Cutan Ocul 23(4), 249-261. |
| ||||||||||||||||||||||||||||||||||||||||||
200331. Ingebrandt S., Yeung C.K., Staab W., Zetterer T., Offenhäusser A. (2003). Backside contacted field effect transistor array for extracellular signal recording. Biosens & Bioelectron. 18, 429-435.
32. *Yeung C.K., Chan K.P., Chiang S.W.Y., Pang C.P., Lam D.S.C. (2003). The toxic and stress responses of cultured human retinal pigmented epithelium (ARPE19) and human glial (SVG) cells in the presence of triamcinolone. Invest Ophthalmol Vis Sci. 44(12), 5293-5300. |
| ||||||||||||
200233. Lauer L., Vogt A., Yeung C.K., Knoll W., Offenhäusser A. (2002). Electrophysiological recordings of patterned rat brain stem slice neurons. Biomaterials. 23(15), 3123-3130.
34. Yeung C.K., McCurrie J.R., Wood D. (2002). Characterisation of the effects of potassium channel modulating agents on mouse intestinal smooth muscle. J. Pharm Pharmacol. 54(3), 425-433. |
| ||||||||||||
200135. *Yeung C.K., Lauer L., Offenhäusser A., Knoll W. (2001). Modulation of the growth and guidance of brain stem neurons using patterned extracellular matrix protein. Neurosci. Lett, 301(2), 147-150.
36. Hudson N.J., Evans A.T., Yeung C.K., Hewitt P.J. (2001). Effect of process parameters upon the dopamine and lipid peroxidation of selected MIG welding fumes as a marker of potential neurotoxicity. Annals Occupational Hygiene. Ann. Occ. Hyg., 45(3), 187-192. 37. *Yeung C.K., Ingebrandt S., Krause M., Offenhäusser A., Knoll W. (2001). Validation of the use of field effect transistors for extracellular recording in pharmacological bioassays. J. Pharmacol. Toxicol. Meth. 45(3), 207-214. 38. Yeung C.K., McCurrie J.R., Wood D. (2001). A simple method to investigate the inhibitory effects of potassium channel openers on gastric emptying in the mouse in vivo. J. Pharmacol. Toxicol. Meth. 45(3), 235-240 39. Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2001). Cardiomyocyte-transistor-hybrids for sensor application. Biosensors & Bioelectronics. 16(7-8), 565-570.
Collaborators (area of collaboration):
(1) John Anthony Rudd (Physiology and Pharmacology of the GI Tract) Professor School of Biomedical Sciences Faculty of Medicine The Chinese University of Hong Kong Shatin, Hong Kong (2) Alaster HY Lau (Physiology and Pharmacology of Mast Cells) Associate Professor School of Biomedical Sciences Faculty of Medicine The Chinese University of Hong Kong Shatin, Hong Kong (3) Mansun Chan (MEA in Bioengineering and Neural Development, Chip Design and Fabrication) Professor Department of Electronic and Computer Engineering The Hong Kong University of Science and Technology Clear Water Bay Kowloon, Hong Kong (4) Sven Ingebrandt (MEA and its application in Neural Development, Chip Design and Fabrication) Professor Department of Informatics and Microsystem Technology, University of Applied Sciences Kaiserslautern, D-66482 Zweibrücken, Germany |
| ||||||||||||||||||||||||||||||
Photos - People associated with the MEA projects (Past and Present)
|
Prof Sven Ingebrandt, who is not only my long-term collaborator but also a close friend of mine, is at the University of Applied Sciences Kaiserslautern; and Dr Jessica Law, who obtained her PhD in 2011, is now working on her postdoc studies in Germany
|
Prof Alaster Lau - Physiology and Pharmacology of Mast Cells
|
Prof. Mansun Chan - MEA in Bioengineering and Neural Development, Chip Design and Fabrication
|