The Mast Cell Project
Mast cells are involved in type-1 allergic responses, typified by the exocytotic release of histamine and other mediators following immunological as well as non-immunological challenges, and this release of histamine appears to be dependent on cytoplasmic calcium concentration ([Ca2+]i). It has long been considered that extracellular Ca2+ influx is important in the mechanism of degranulation, with intracellular Ca2+ stores also playing a pivotal role in the initial increase of cytosolic Ca2+. While Ca2+ entry and Ca2+ release–activated Ca2+ (CRAC) channels are obviously important in mast cell activation, ionic gradients of potassium (K+) and chloride (Cl−) are likely to also play important roles in mast cell secretory responses through their influence on membrane potential and thus Ca2+ influx. Available evidence has revealed that the activation of the outward K+ current correlates with the activation of CRAC current in both time and amplitude and that a Ca2+-activated K+ (KCa) current enhances the release of mediators. Furthermore, in contrast to excitable cells, mast cell membrane hyperpolarisation is required to support Ca2+ entry.
MEA vs Patch-Clamp
Since there is an intimate relationship between K+ and Ca2+ levels and mast cell degranulation, many studies have utilised conventional electrophysiological techniques, such as patch-clamp, to investigate their contributions to mast cell activation. While the use of patch-clamp has proven to be invaluable in the evaluation of drug action on mast cells, the technique itself is difficult and time-consuming to perform. In view of the need to measure electrophysiological changes of mast cells both quickly and efficiently as well as to provide a convenient means of screening a large amount of potential anti-allergic compounds on mast cell activation, we propose the use of microelectrode arrays (MEAs) in future allergy screening.
Method and Approach
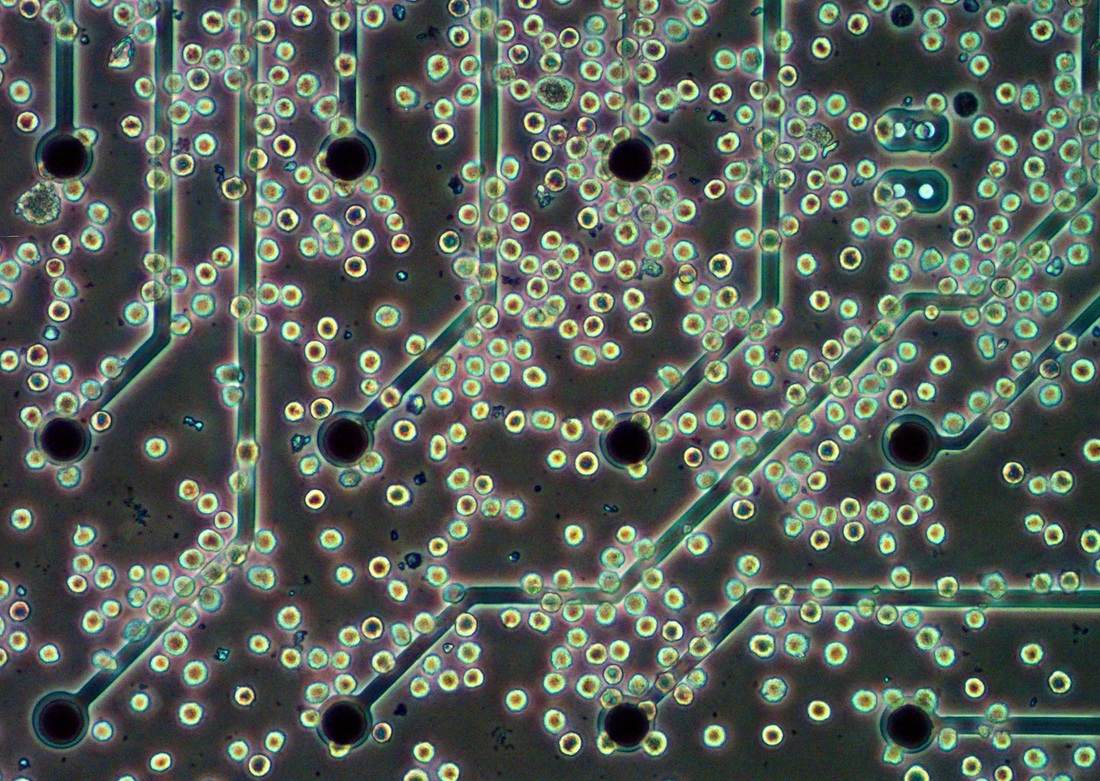
Most currently available MEA systems, including our own, are only measuring field potentials of cardiac or neuronal cell monolayers in the order of several 100’s of μV to 1 or 2 mV at best. It was therefore surprising to find that upon stimulation by a secretagogue, the recorded peritoneal mast cell field potentials were in the range of 4 to 6 mV and even up to more than 8 mV had been recorded in some occasions. This shows the present system is suitable for electrophysiological studies of non-excitable cells such as mast cells. Moreover, the fact that it could measure electrophysiological changes without doing so invasively means that the cellular homeostasis is preserved.
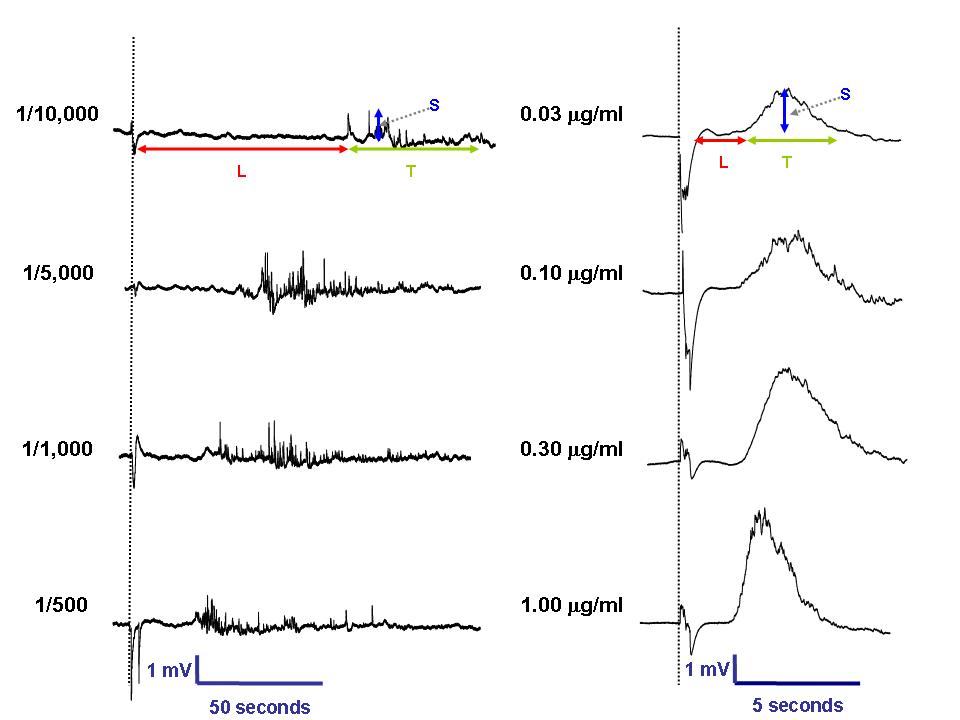
Concentration-dependent changes in exFPs of the RPMC by the presence of anti-IgE (1/10,000-1/500, left panel) and compound 48/80 (0.03-1µg/ml, right panel) were observed.
L = Latent, the onset time of exFP (sec) T = Temporal, the response time of exFP (sec) S = spatial, the field potential of exFP (mV) |
The Relative Contributions of Different Ions Can Be Characterised
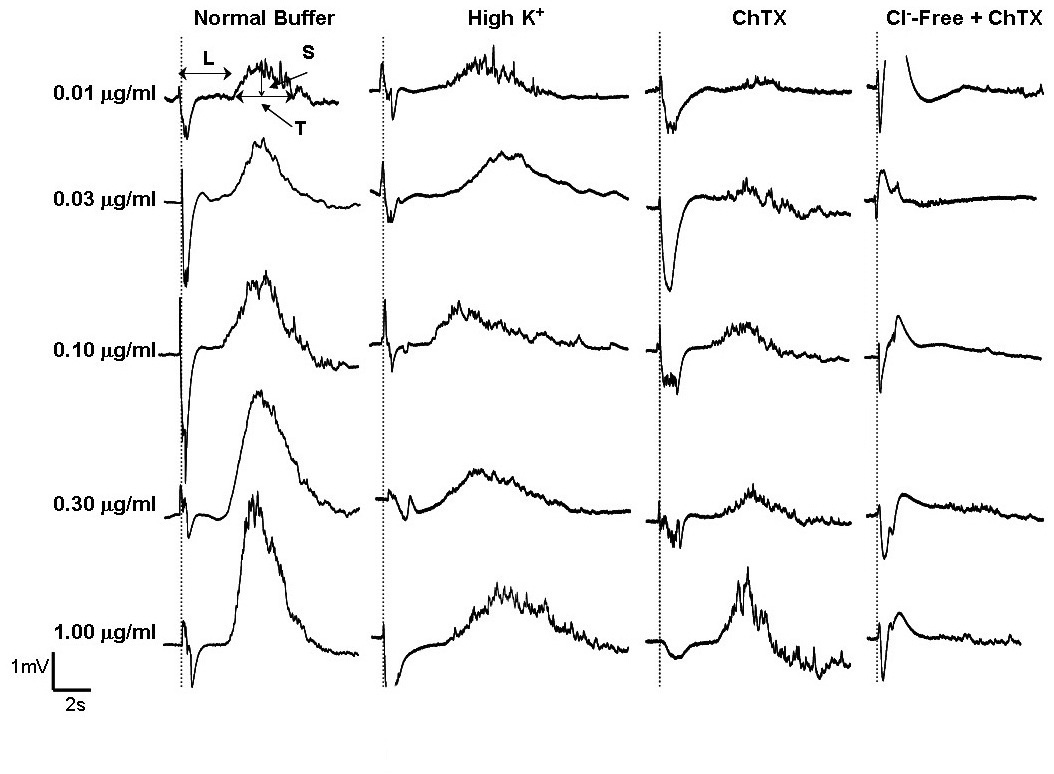
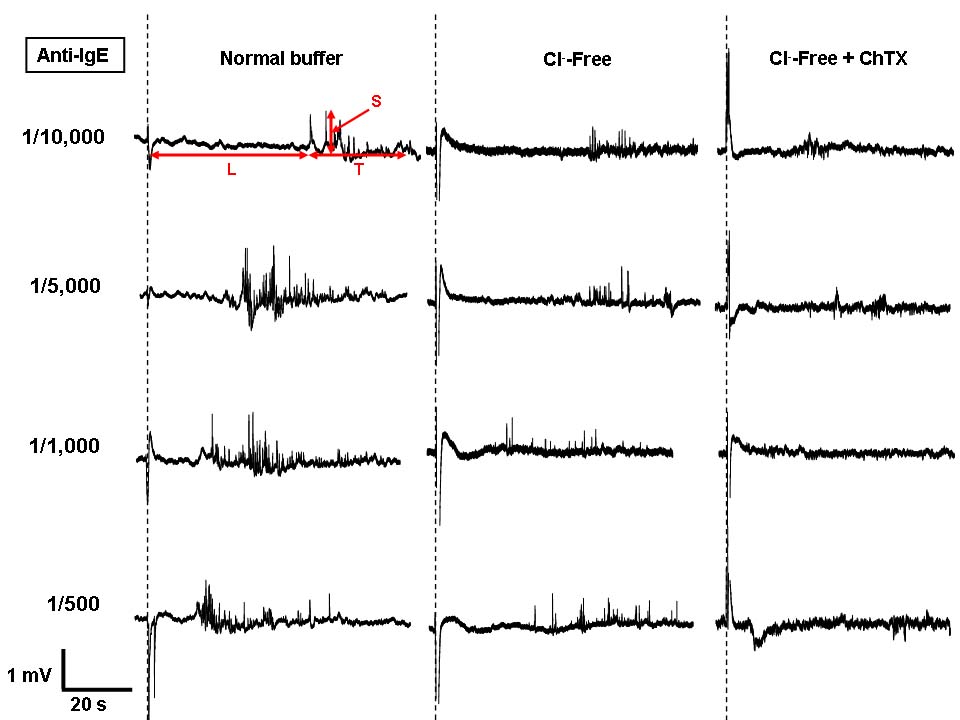
Field potential recordings of mast cells in the presence of compound 48/80 (0.01-1mg/ml, left panel) or anti-IgE (1/10,000-1/500, right panel) were compared with those obtained when cells were exposed to Cl--free or Cl--free containing charybdotoxin (ChTX, 100nM) buffer. The latent period (L), spatial (S), and temporal (T) measurements are indicated. The large artefacts along the doted lines indicate the moment when compound 48/80 or anti IgE was added.
Ionic Components of Mast Cell Degranulation
Our data thus far have revealed that the recorded positive signal shape comprises of at least two components: i) K+ efflux, which probably includes the activation of inwardly rectifying K+ and KCa channels, and ii) an additional hyperpolarising potential that is unrelated to K+ channel activation. The K+ efflux component is partially inhibited by high K+ or ChTX, and the remaining hyperpolarising component is virtually completely eliminated when extracellular Cl− is also removed from the ChTX-containing buffer. Our MEA system is probably the first to be able to monitor the combined changes of K+ and Cl− potentials of mast cells.
References
Law J.K.Y., *Yeung C.K., Wan S.P., Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2011). The significance of chloride in the inhibitory action of disodium cromoglycate on immunologically-stimulated rat peritoneal mast cells. Biochimica et Biophysica Acta. 1810, 867-874.
*Yeung C.K., Law J.K.Y., Sam S.W, Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2009). Modulatory action of potassium channel openers on field potential and histamine release from rat peritoneal mast cells. Can.J. Physiol. Pharmacol. 87(8):624-32
*Yeung C.K., Law J.K.Y., Sam S.W., Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2008). The use of microelectrode array (MEA) to study rat peritoneal mast cell activation. J Pharmacol Sci. 107, 201-212.
*Yeung C.K., Law J.K.Y., Sam S.W, Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2009). Modulatory action of potassium channel openers on field potential and histamine release from rat peritoneal mast cells. Can.J. Physiol. Pharmacol. 87(8):624-32
*Yeung C.K., Law J.K.Y., Sam S.W., Ingebrandt S., Lau H.Y.A., Rudd J.A., Chan M. (2008). The use of microelectrode array (MEA) to study rat peritoneal mast cell activation. J Pharmacol Sci. 107, 201-212.
Click to see the other MEA projects |