The Cardiovascular Project
This is one of the major projects involving the use of the Microelectrode Array (MEA).
Even in culture (dissociated heart cells from animals), cardiac myocytes will form viable network (beating syncytium) within a couple of days. Once the cells are connected again through gap junctions, the entire layer of cells will contract spontaneously; i.e. autorhythmic.
Cultured cardiac myocytes will form a syncytium of beating network in a few days. |
A pacemaker region, which drives the entire syncytium and governs its autorhythmicity, can be found in cardiac cultures. |
See how a mature syncytium of heart cells beat around an MEA recording electrode. |
The propagation of cardiac field potential across the entire 8 x 8 electrode substrate can be visualised. |
The same propagation over the 8 x 8 grid can also be viewed in 3D.
|
The conduction velocity of cardiac myocytes on an MEA can be determined. |
|
|
|
The Significance of the Signal Components
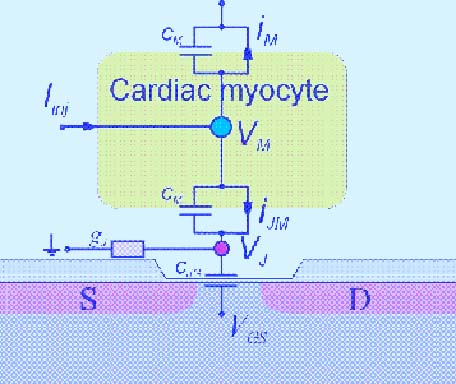
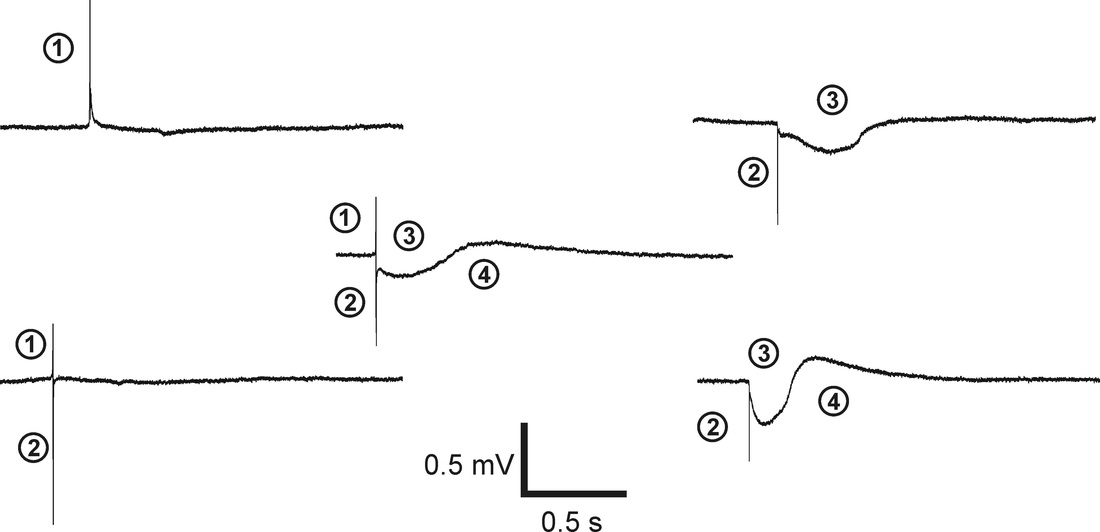
The extracellular signal shapes of cardiac myocytes are composed of several signal components, and the interpretations of signal shapes have been described (Yeung et al., 2007). These signal shape components are outlined as follows:
1. The fast up-spike is related to the depolarisation of the cell membrane. The amplitude of this peak is proportional to the first derivative of the time-dependent membrane voltage. 2. The fast down-spike is related to the Na+ currents through the small cleft between the membrane and the sensor surface.
3. The slow negative signal component is mainly the result of calcium (Ca2+) influx.
4. The slow positive signal is the result of the repolarising potassium (K+) efflux.
1. The fast up-spike is related to the depolarisation of the cell membrane. The amplitude of this peak is proportional to the first derivative of the time-dependent membrane voltage. 2. The fast down-spike is related to the Na+ currents through the small cleft between the membrane and the sensor surface.
3. The slow negative signal component is mainly the result of calcium (Ca2+) influx.
4. The slow positive signal is the result of the repolarising potassium (K+) efflux.
The purpose of this study is to establish an in vitro experimental platform of cultured cardiacmyocytes on the MEA and to use this system to monitor the electrophysiological changes of the entire syncytium due to acute hypoxia. The obtained electrophysiological observations are compared with the presently known physiological changes, such as lactate concentration, pH, and osmolarity, of the heart under hypoxia. This study aims to show that such a cell-integrated electronic system may be useful for a variety of pharmacological studies of heart.
|
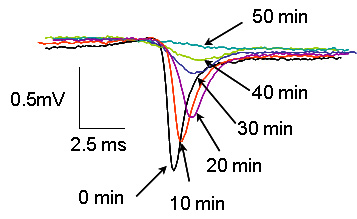
The figure shows that the rate of change of depolarisation from 5 to 50 min. The sodium signal magnitudes reduced and their durations lengthened progressively from 5 to 50 min of hypoxia (component 2), indicating the electrochemical gradient of sodium during these periods was gradually being reduced due to hypoxic insult. Normoxic traces across the same time points are shown as comparison. Signal components (3) and (4) cannot be seen at this time scale.
|
Anaesthesia and Cardioprotection - A State of Having a 'Memory'
At present, we want to investigate the Involvement of Potassium Channels in Anaesthetic-Induced Preconditioning.
It is known that pretreatment with isoflurane attenuates oxidative stress induced cell death. Over the past decade, it has been shown that ischaemic cardiac injury can be substantially alleviated by exposing the heart to pharmacological agents, such as volatile anaesthetics, before occurrence of ischaemia-reperfusion. A hallmark of this preconditioning phenomenon is its ‘memory’; i.e. cardioprotective effects persist even after removal of preconditioning stimulus.
It has long been suspected that anaesthetic agents prolong cardiac repolarisation by blocking ion currents; however, the clinical relevance of this blockade in subjects with reduced repolarisation reserve is unknown. Anaesthetic-induced preconditioning (APC) by isoflurane decreases sensitivity of the sarcolemmal KATP channel to inhibition by adenosine 5'-triphosphate (ATP) and decreases adenosine 5'-diphosphate (ADP) sensitivity. These effects persist even after discontinuation of the anaesthetic, suggesting a possible novel factor that may contribute to the mechanism of early memory of APC.
The present study aims to characterise the involvement(s) of ion channels, especially potassium channels, in anaesthesia-induced cardioprotection.
It is known that pretreatment with isoflurane attenuates oxidative stress induced cell death. Over the past decade, it has been shown that ischaemic cardiac injury can be substantially alleviated by exposing the heart to pharmacological agents, such as volatile anaesthetics, before occurrence of ischaemia-reperfusion. A hallmark of this preconditioning phenomenon is its ‘memory’; i.e. cardioprotective effects persist even after removal of preconditioning stimulus.
It has long been suspected that anaesthetic agents prolong cardiac repolarisation by blocking ion currents; however, the clinical relevance of this blockade in subjects with reduced repolarisation reserve is unknown. Anaesthetic-induced preconditioning (APC) by isoflurane decreases sensitivity of the sarcolemmal KATP channel to inhibition by adenosine 5'-triphosphate (ATP) and decreases adenosine 5'-diphosphate (ADP) sensitivity. These effects persist even after discontinuation of the anaesthetic, suggesting a possible novel factor that may contribute to the mechanism of early memory of APC.
The present study aims to characterise the involvement(s) of ion channels, especially potassium channels, in anaesthesia-induced cardioprotection.
References
Law J.K.Y., *Yeung C. K., Frisch J., Knapp S., Ingebrandt S., Rudd J. A., Chan M. (2012). Cardioprotective effects of potassium channel openers on rat atria and isolated hearts under acute hypoxia. J Phys Pharm Adv.2:41-48.
Law J.K.Y., *Yeung C.K., Yiu K.L., Rudd J.A., Ingebrandt S., Chan M. (2010). A study of the relationship between pharmacological preconditioning and adenosine triphosphate-sensitive potassium (KATP) channels on cultured cardiomyocytes using the microelectrode array. J Cardiovasc Pharmacol 56, 60-68.
Law J.K.Y., *Yeung C.K., Hofmann B., Ingebrandt S., Rudd J.A., Offenhäusser A., Chan M. (2009). The use of microelectrode array (MEA) to study the protective effects of potassium channel openers on metabolically-compromised HL-1 cardiomyocytes. Physiol. Meas. 30, 155-167.
Yeung C.K., Sommerhage F., Wrobel G., Law J.K.Y., Offenhäusser A., Rudd J.A., Ingebrandt S., Chan M. (2009). To establish a pharmacological experimental platform for the study of cardiac hypoxia using the microelectrode array. J. Pharmacol Toxicol. Meth. 59, 146-152.
Yeung C.K., Sommerhage F., Offenhäusser A., Chan M., Ingebrandt S. (2007). Drug profiling using planar microelectrode arrays. Anal Bioanal Chem. 387, 2673-2680.
Law J.K.Y., *Yeung C.K., Yiu K.L., Rudd J.A., Ingebrandt S., Chan M. (2010). A study of the relationship between pharmacological preconditioning and adenosine triphosphate-sensitive potassium (KATP) channels on cultured cardiomyocytes using the microelectrode array. J Cardiovasc Pharmacol 56, 60-68.
Law J.K.Y., *Yeung C.K., Hofmann B., Ingebrandt S., Rudd J.A., Offenhäusser A., Chan M. (2009). The use of microelectrode array (MEA) to study the protective effects of potassium channel openers on metabolically-compromised HL-1 cardiomyocytes. Physiol. Meas. 30, 155-167.
Yeung C.K., Sommerhage F., Wrobel G., Law J.K.Y., Offenhäusser A., Rudd J.A., Ingebrandt S., Chan M. (2009). To establish a pharmacological experimental platform for the study of cardiac hypoxia using the microelectrode array. J. Pharmacol Toxicol. Meth. 59, 146-152.
Yeung C.K., Sommerhage F., Offenhäusser A., Chan M., Ingebrandt S. (2007). Drug profiling using planar microelectrode arrays. Anal Bioanal Chem. 387, 2673-2680.
Click to see the other MEA projects |