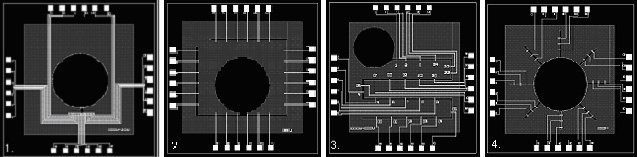
The Microelectrode Array (MEA)
We currently have three different MEA systems in our laboratory: (1) a 60-channel MEA1060 system, was obtained following a project grant being successfully awarded in 2010. It is now mainly used for gastrointestinal slow waves recording; (2) a newer 60-channel MEA2100 system was built for measuring spiking activities using cell culture or acute brain slices; the 64-channel system originally designed and built by our collaborators in Germany (Max-Planck-Institute for Polymer Research).
|
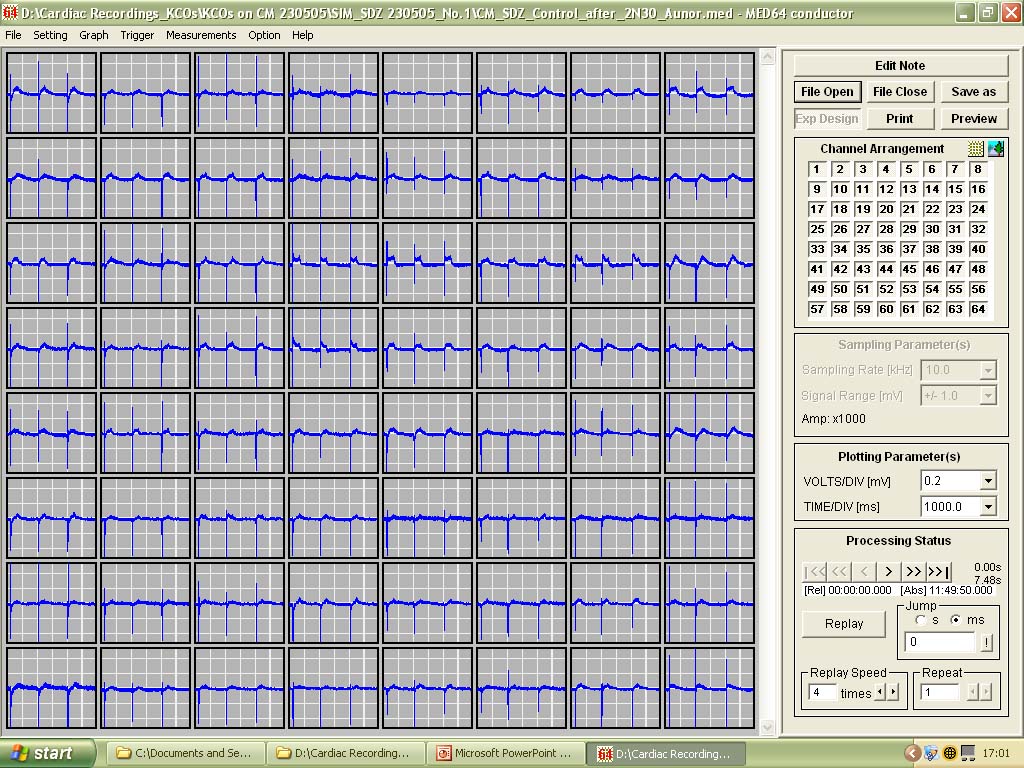
The MEA1060 systems for gastrointestinal slow wave recordings.
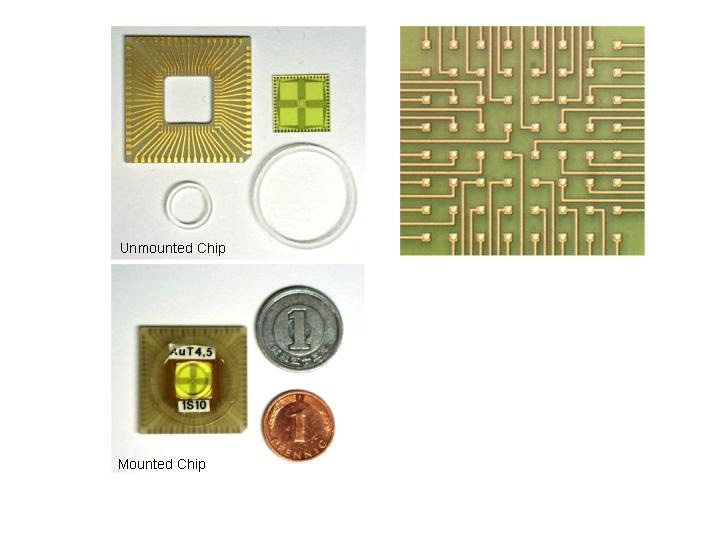
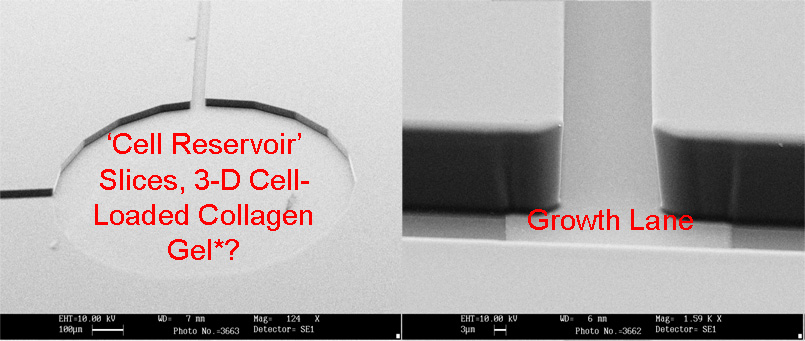
MEA Chip - Individual Components and Channel Organisation |
The MEA2100 system for spiking activity recordings.
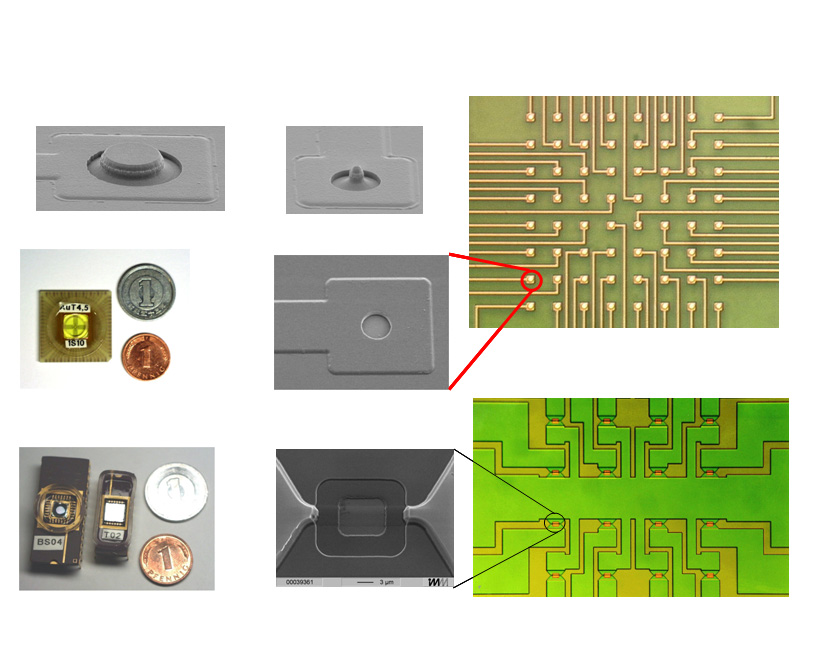
MEA Chip - Electron micrographs showing the electrode shapes and relative sizes of MEA compared to field effect transitor (bottom right) |
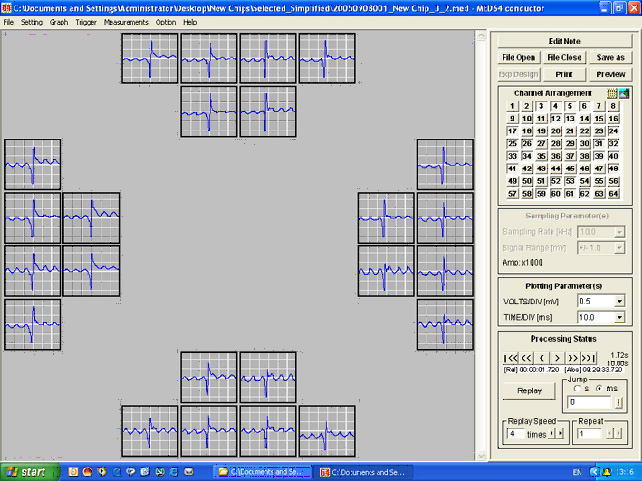
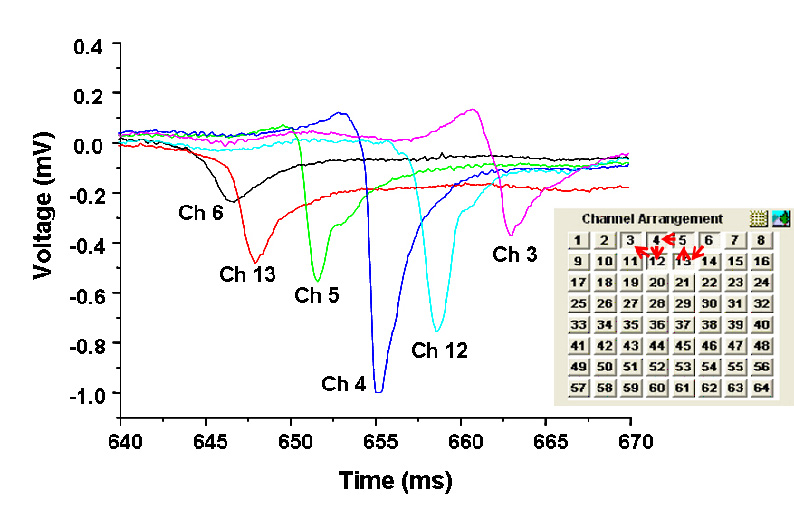
'Population Thinking' - As opposed to single or even multiple patch-clamp recordings, all 64 channels (with a potential for recording 64 cells simultaneously) can be displayed at the same time in different ways depending on the types of data being recorded.
One of our aims is to design and fabricate our own MEAs for specific purposes. Some of these designs are shown below
Figure shows recordings along the top 6 channels |
We aim to acheive:Formation of cellular networks (guided or non-guided neural nets) or cardiac myocytes on the MEA.
Recordings of cellular signals (field potentials). Stimulation of cellular response using ‘stimulation electrodes’. Identification of signal shapes, which reflect the physiological or pathophysiological states of the recorded cells - i.e. to generate 'electrophysiologically recognisable patterns' or some sort of 'signatures' corresponding to specific physiological states. |
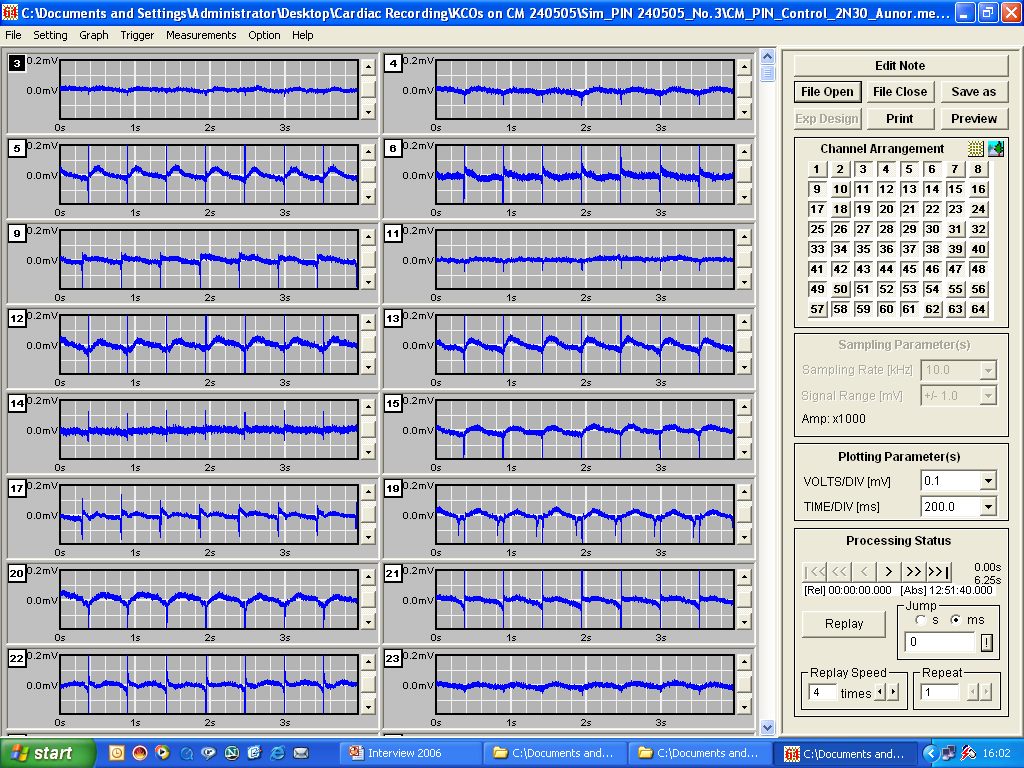
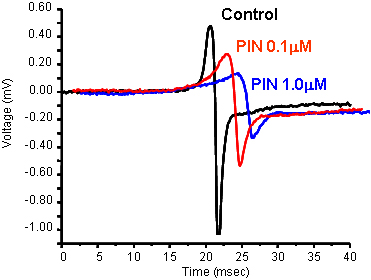
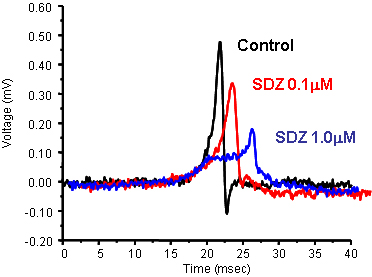
Quantitative PharmacologyJust as performing isolated organ experiments, the MEA can be used to carry out concentration-response curves.
PIN (pinacidil) and SDZ (SDZ PCO400) are potassium channel openers that act on two different KATP channels - sarcolemmal KATP channels and mitochondrial KATP channels, respectively
|
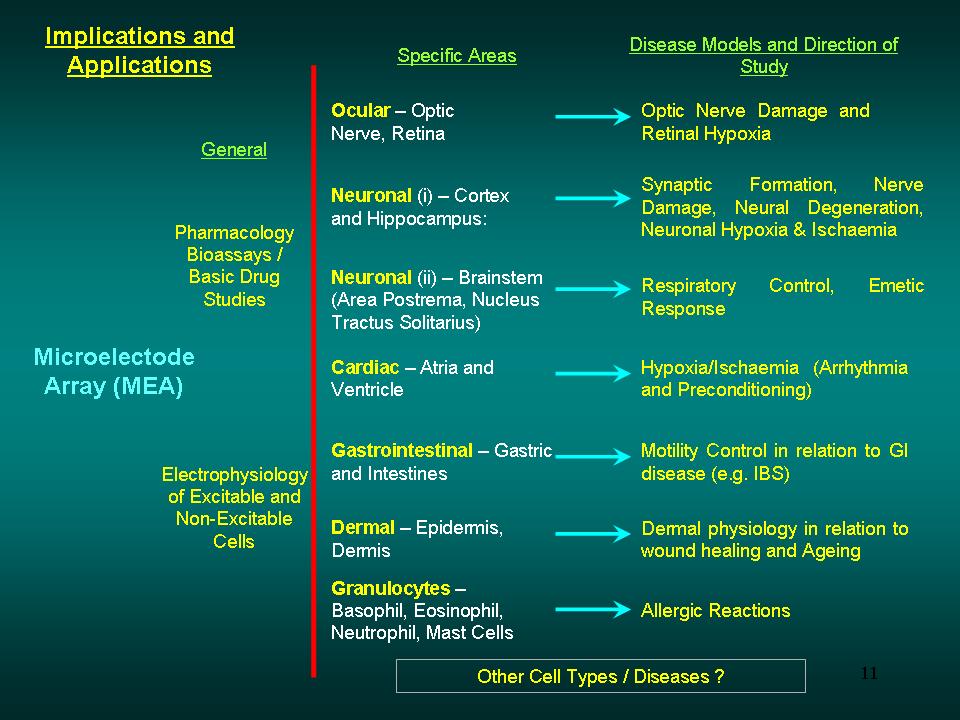
Applications of MEA
While we are primarily focussing on the heart, the brain, mast cells, and RGCs, there are other areas that the MEA can be used to answer some very important physiological questions. For example, gastrointestinal motility is governed by very intricate ionic movements across the cell membrane; the understanding of which can help us understand a variety of GI diseases, such as irritable bowel syndrome.
|
References
Guo J., Yuan J., Huang J., Law J.K.Y., Yeung C.K., Chan M. (2012). 32.9nV/rt Hz – 60.6dB THD Dual-Band Micro-Electrode Array Signal Acquisition IC. IEEE Journal of Solid-State Circuits. 47, 1209-1220.
Law J.K.Y, *Yeung C.K., Lin Li., Rudd J.A., Ingebrandt S., Chan M. (2012). The use of SU-8 topographical guided microelectrode array in measuring extracellular field potential propagation. Ann Biomed Eng. 40:619-627.
Guo J., Yuan J., Huang J., Law J.K.Y., Yeung C.K., Chan M. (2011). Highly accurate dual-band cellular field potential acquisition for brain-machine interface. IEEE Journal on Emerging and Selected Topics in Circuits and Systems. 1, 461-468
Yeung C.K., Sommerhage F., Offenhäusser A., Chan M., Ingebrandt S. (2007). Drug profiling using planar microelectrode arrays. Anal Bioanal Chem. 387, 2673-2680.
Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2005). Neuron-Transistor Coupling: Interpretation of individual extracellular recorded signals. European Biophysics J, 34, 144-154.
Ingebrandt S., Yeung C.K., Staab W., Zetterer T., Offenhäusser A. (2003). Backside contacted field effect transistor array for extracellular signal recording. Biosens & Bioelectron. 18, 429-435.
Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2001). Cardiomyocyte-transistor-hybrids for sensor application. Biosensors & Bioelectronics. 16(7-8), 565-570.
*YeungC.K., Ingebrandt S., Krause M., Offenhäusser A., Knoll W. (2001). Validation of the use of field effect transistors for extracellular recording in pharmacological bioassays. J. Pharmacol. Toxicol. Meth. 45(3), 207-214.
Law J.K.Y, *Yeung C.K., Lin Li., Rudd J.A., Ingebrandt S., Chan M. (2012). The use of SU-8 topographical guided microelectrode array in measuring extracellular field potential propagation. Ann Biomed Eng. 40:619-627.
Guo J., Yuan J., Huang J., Law J.K.Y., Yeung C.K., Chan M. (2011). Highly accurate dual-band cellular field potential acquisition for brain-machine interface. IEEE Journal on Emerging and Selected Topics in Circuits and Systems. 1, 461-468
Yeung C.K., Sommerhage F., Offenhäusser A., Chan M., Ingebrandt S. (2007). Drug profiling using planar microelectrode arrays. Anal Bioanal Chem. 387, 2673-2680.
Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2005). Neuron-Transistor Coupling: Interpretation of individual extracellular recorded signals. European Biophysics J, 34, 144-154.
Ingebrandt S., Yeung C.K., Staab W., Zetterer T., Offenhäusser A. (2003). Backside contacted field effect transistor array for extracellular signal recording. Biosens & Bioelectron. 18, 429-435.
Ingebrandt S., Yeung C.K., Krause M., Offenhäusser A. (2001). Cardiomyocyte-transistor-hybrids for sensor application. Biosensors & Bioelectronics. 16(7-8), 565-570.
*YeungC.K., Ingebrandt S., Krause M., Offenhäusser A., Knoll W. (2001). Validation of the use of field effect transistors for extracellular recording in pharmacological bioassays. J. Pharmacol. Toxicol. Meth. 45(3), 207-214.