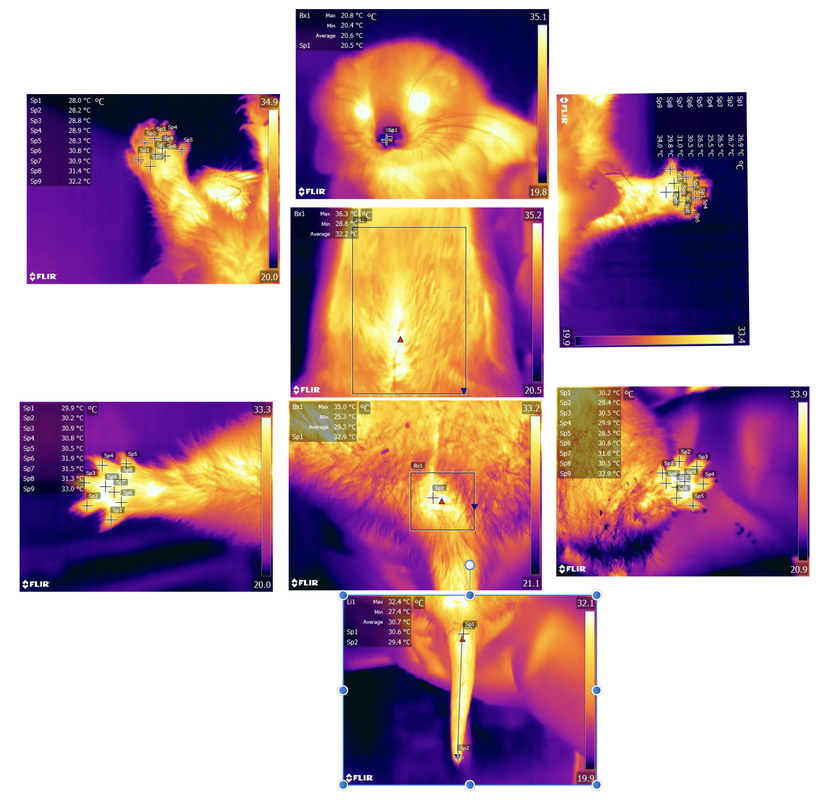
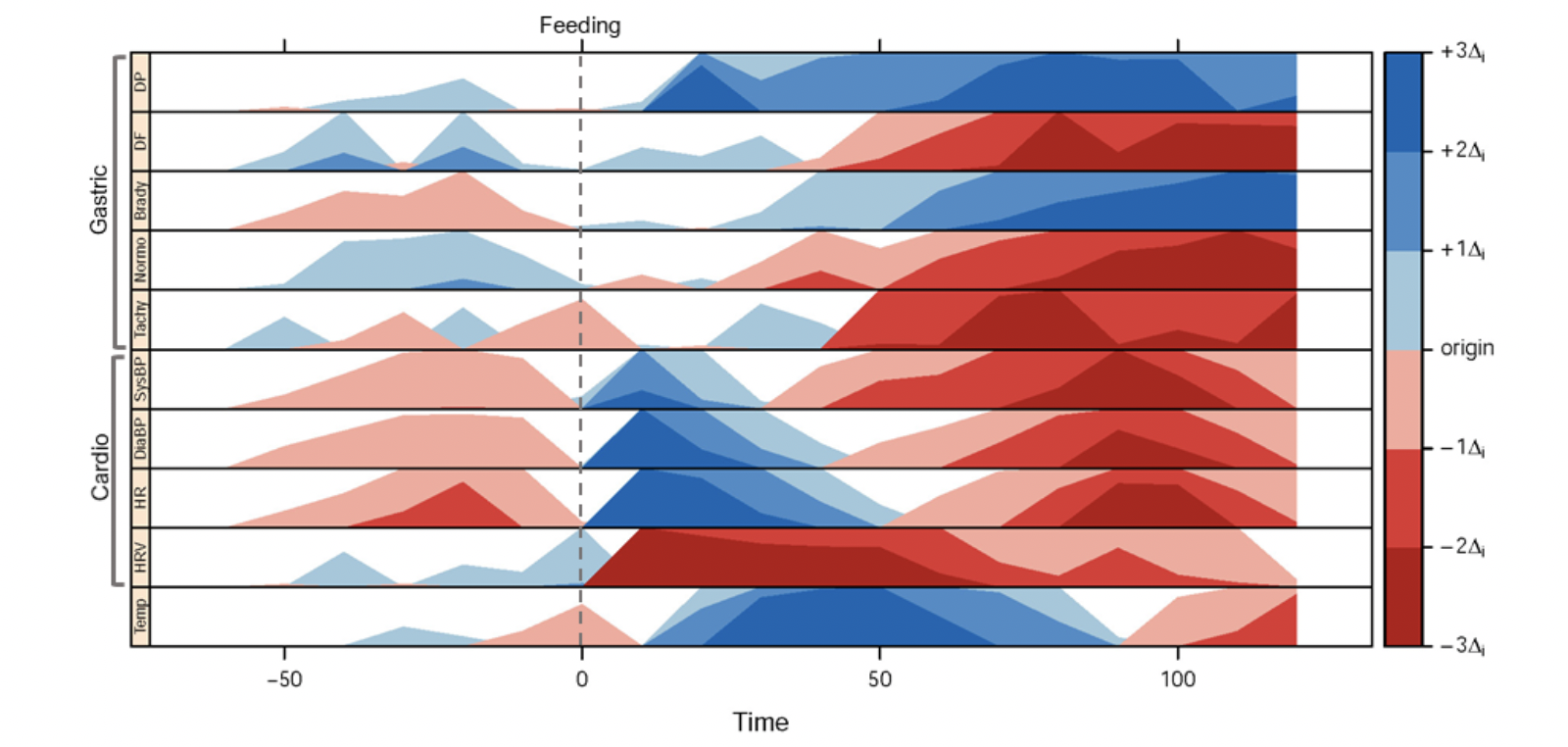
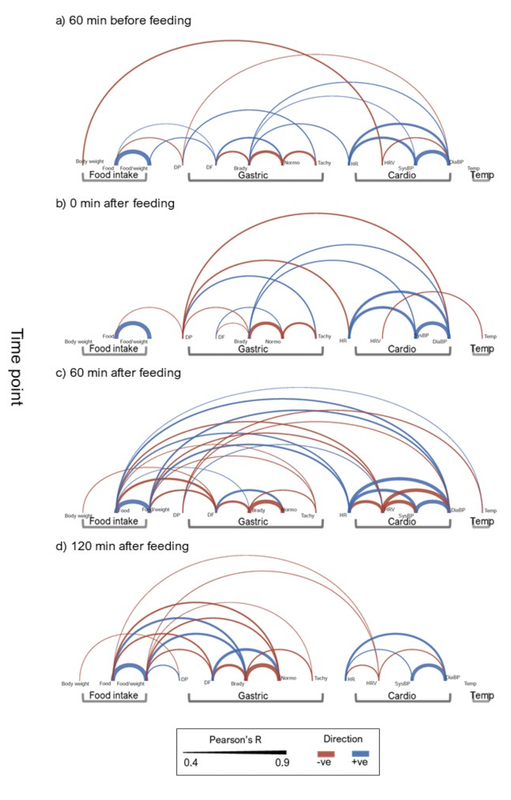
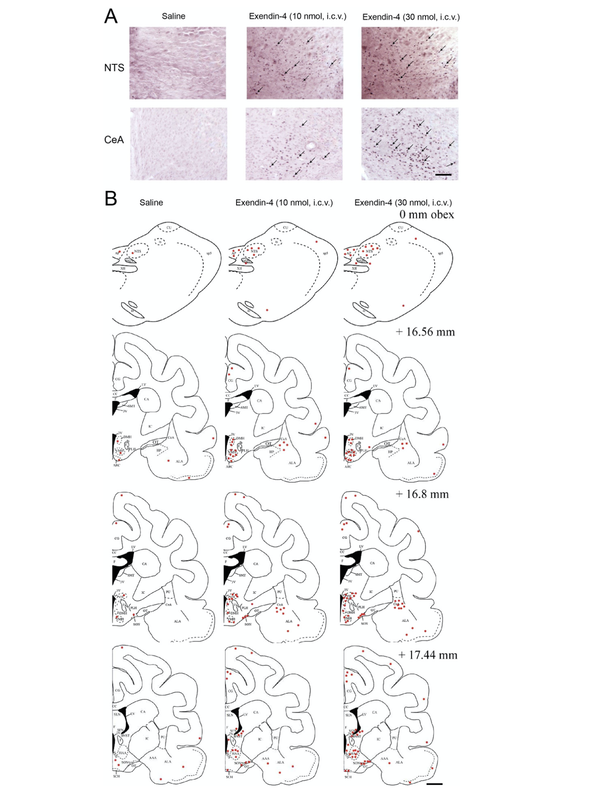
Physiological Changes Indicative of 'Nausea'
|
|
Behavioural Data
Physiological Data
|
Plasma/Tissue Biomarker Data/C-Fos
Validation Relative to Off-Target Effects
|
Physiological Changes Indicative of 'Nausea': PCIN
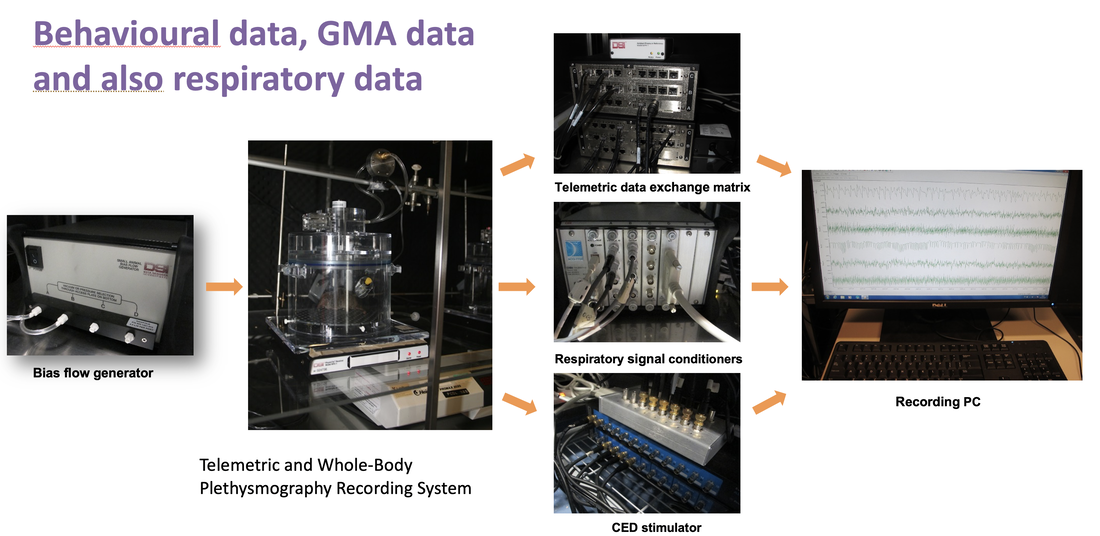
Radiotelemetry
|
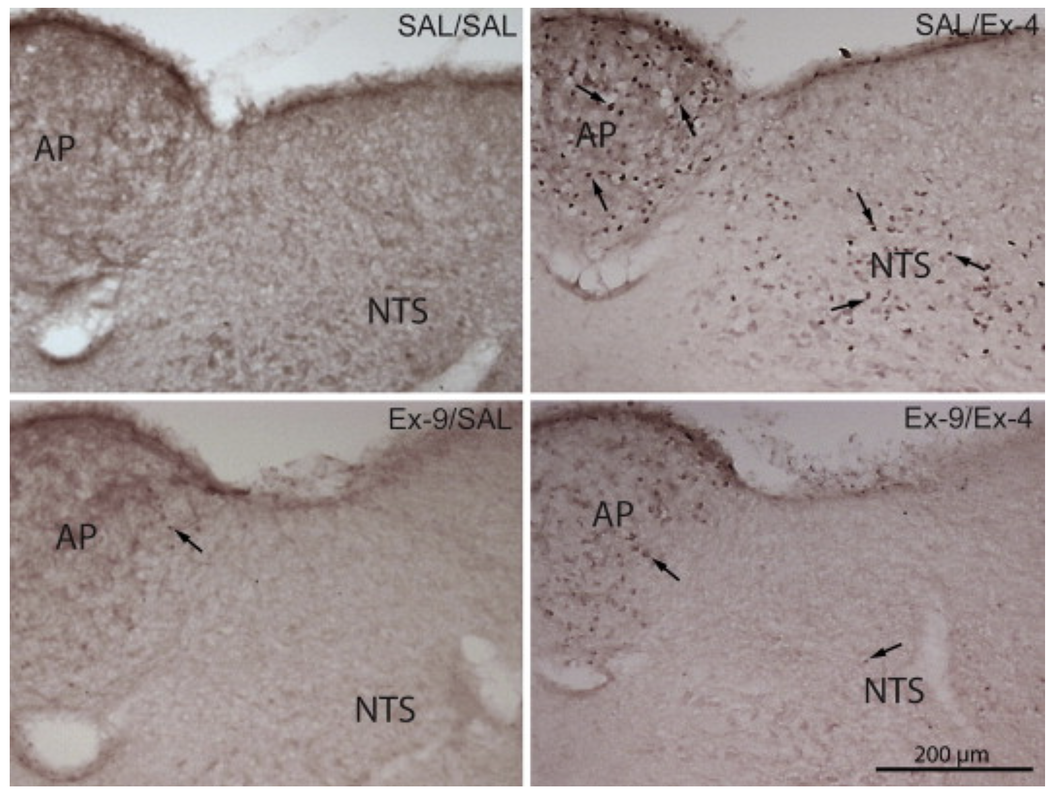
c-Fos Patterns
|
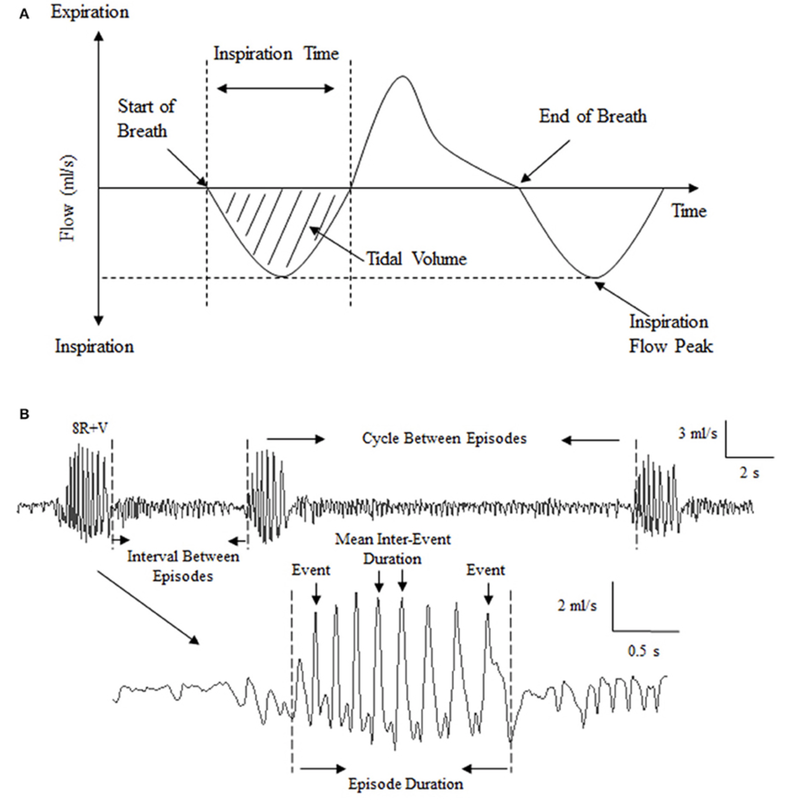
Behaviour & Respiratory
|